What is calcite?
Calcite is an important calcium carbonate mineral with rich colors and diverse crystal forms. It is called the mineral of a thousand crystals. Calcite is mainly composed of limestone and marble and is one of the most common minerals in the earth's crust.

How is calcite formed?
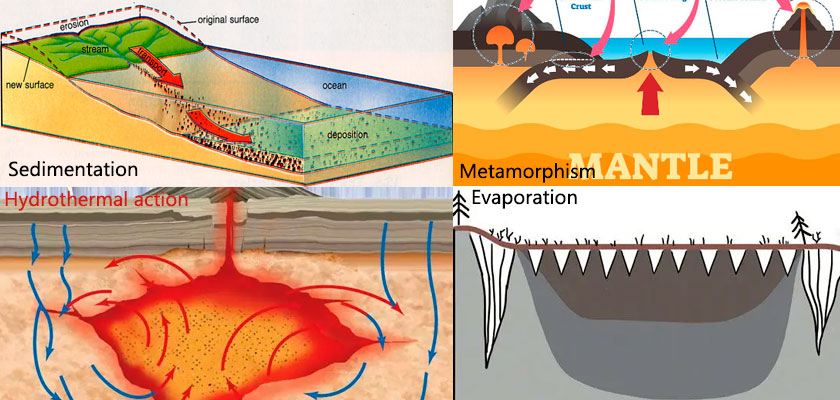
Calcite is mainly composed of calcium carbonate. It can be formed in a variety of geological environments, including sedimentation, hydrothermal action, biological action and metamorphism.
Sedimentation: Marine organisms such as corals and foraminifera absorb calcium carbonate to form skeletons, which are deposited on the seabed after death. After compaction and cementation, limestone is formed, and the main component is calcite.
Biological action: After the death of corals, shellfish, marine microorganisms, etc., their skeletons and shells are deposited on the seabed. After diagenesis, they gradually become biological limestone, in which calcite is the main mineral.
Hydrothermal action: Hydrothermal fluids flow along rock cracks, and calcite is precipitated after cooling to form hydrothermal veins. Hydrothermal fluids react chemically with surrounding rocks to form calcite.
Metamorphism: When limestone or chalk undergoes metamorphism under high temperature and pressure, it recrystallizes and forms marble or other metamorphic rocks.
Evaporation: In arid areas, water in lakes or shallow seas evaporates, causing calcium carbonate to be supersaturated and precipitate to form calcite, such as the Dead Sea and the Great Salt Lake.
Crystal form of calcite
Calcite crystals mainly present a trigonal crystal structure, and common forms are rhombohedron, scalenohedron, columnar, plate-like, etc. It has a significant birefringence phenomenon, and a double image can be seen through the crystal.

Properties of calcite
Physical properties: Usually colorless or white, but can also be gray, yellow, green, blue, etc. It has a glassy luster and a Mohs hardness of 3. It has complete rhombohedral cleavage and is easy to crack in a specific direction.
Optical properties: Calcite is transparent to translucent, has a high birefringence, and is often used in optical instruments.
Chemical properties: The chemical formula of calcite is CaCO₃. It will react violently with dilute hydrochloric acid and release carbon dioxide. This is an important method to identify calcite.

Uses of calcite
Construction uses: Calcite is an important raw material for making cement and lime, and is widely used in infrastructure construction such as buildings, roads, bridges, etc.
Decorative carving: Marble formed by the metamorphosis of calcite is often used in architectural decoration, such as floors, walls, sculptures, etc.
Industrial uses: It is used as a filler in the plastic, paint, rubber and paper industries to improve product performance. At the same time, quicklime is generated after calcination and is used in construction and chemical industry.
Agricultural uses: Calcite powder can be used to adjust the pH value of acidic soil and improve acidic soil. It is added to livestock feed as a calcium source to enhance animal bone health.
Chemical uses: Calcite can be used to neutralize the acidic components in industrial wastewater and exhaust gas. It can also be used to produce chemical products such as calcium carbonate and calcium chloride.
Optical uses: Calcite has birefringence properties and is used to make polarizing prisms and other optical instruments.
Medical use: Calcite, as a source of calcium, can be used to neutralize stomach acid, manufacture medicines and health products, etc.
Gemstone collection: Calcite has rich colors and various crystal forms, and is often used to make low-end jewelry, ornamental stones, mineral specimens, etc.

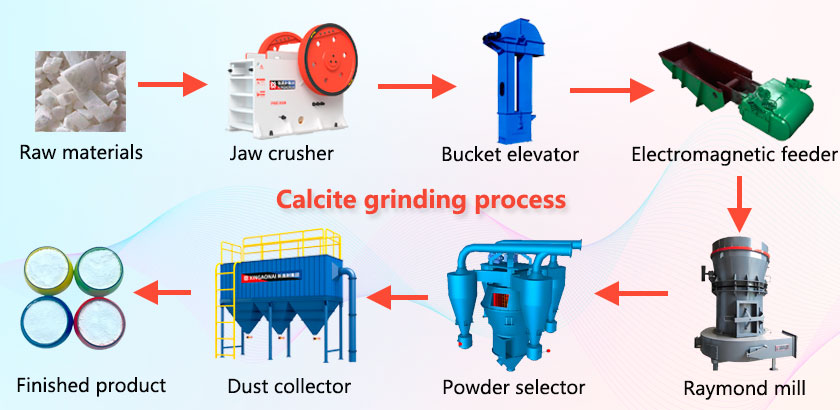
Calcite grinding process flow
Main equipment: jaw crusher, bucket elevator, electromagnetic feeder, Raymond mill, powder selector, dust collector, etc.
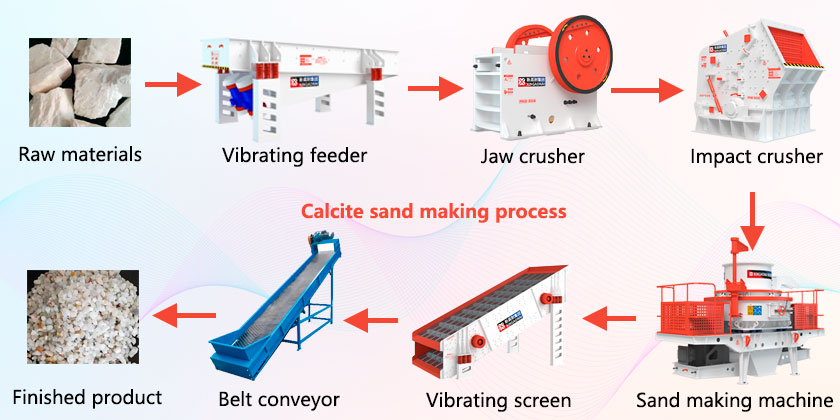
Calcite sand making process flow
Main equipment: vibrating feeder, jaw crusher, impact crusher, sand making machine, vibrating screen, belt conveyor, etc.
Author:[Xingaonai]
Article Title: What is calcite? What is calcite used for?
Reprint URL: https://www.xgncrusher.com/Industralnews/what-is-calcite-what-is-calcite-used-for.html